Side Projects / Biofuels
This work was part of a class project for environmental engineering in 2007. We wanted to summarize the situation for the world’s energy consumption, and investigate realistically how biofuels (solar energy stored in plants) could help in covering some energy needs. Below, you will find the abstract and several slides explaining the findings.
Abstract
This report is aimed at providing a summary of the field of biofuels: the production of liquid fuels from plants. Biofuels are not aiming at solving the world energy problem, but rather at providing a viable alternative to the transportation fuels which are presently derived almost in their entirety from imported oil. Rising oil prices, instabilities in the oil-producing regions of the world and greenhouse gas emissions from fossil fuels provide the motivation behind a field in ferment.
As opposed to other renewable intermittent energy technologies such as photovoltaic cells and wind farms, which require (currently inefficient) electrical storage mechanisms in order to function reliably over long periods of time, plants absorb solar energy and store it chemically inside their biomass. It is estimated by our report that 1TW of average power is stored into available for biofuel production biomass in the United States only (the global power consumption is during the year 2007 at 15TW). Even if a small fraction of that stored energy can be retrieved from the biomass, a significant portion of motor fuel could be replaced.
The first chapter summarizes the present global situation in terms of energy demand, CO2 emissions and oil consumption. Chapter 2 provides a basic background on biofuels and examines their potential from an energy perspective. Chapter 3 provides an overview of the biofuel landscape in the United States, which is currently relying on ethanol fuel derived from corn kernels to provide 3% of its transportation fuels, although this type of ethanol could not be expanded into large scale. Chapter 4 examines the details of producing ethanol from the cellulose molecules that comprise the plant walls, which, if harnessed properly, can have much higher efficiencies and energy outputs than crop-derived ethanol because it can consume non-traditional biomass which is not used directly for other purposes. Chapter 5 describes briefly other biofuel production techniques, such as Biodiesel (popular in Germany), sugarcane-derived ethanol (successful in Brazil), Biobutanol and algae cultivation. Finally, we summarize the report in chapter 6.
Henry Ford himself when he build the first motor, he used biofuels (vegetable oil) to run his engines initially. Of course later he realized that fuel from oil was much more powerful than biofuels at the time (still is), and he switched to oil, for never to return to biofuels.
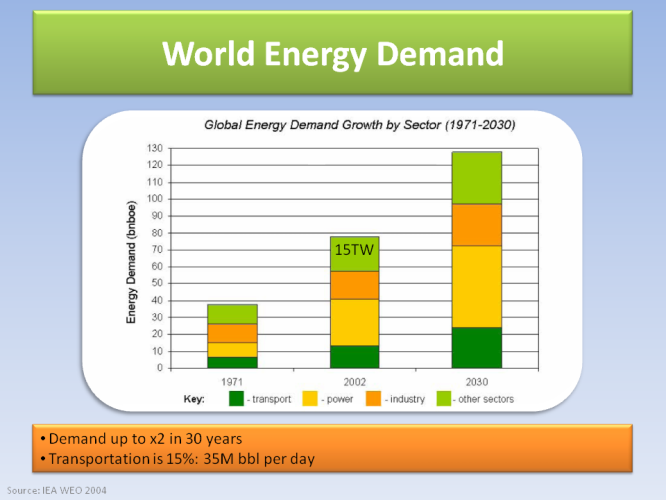
To put things in perspective, we need to know about world energy consumption. It doubled in 30 years, and it will almost double again in another 30 years. By 2030, we will need 30 TW of average power, from which 15% will be transportation energy, mostly oil. Currently energy for transport is 35 million barrels of oil (bbl) PER DAY.
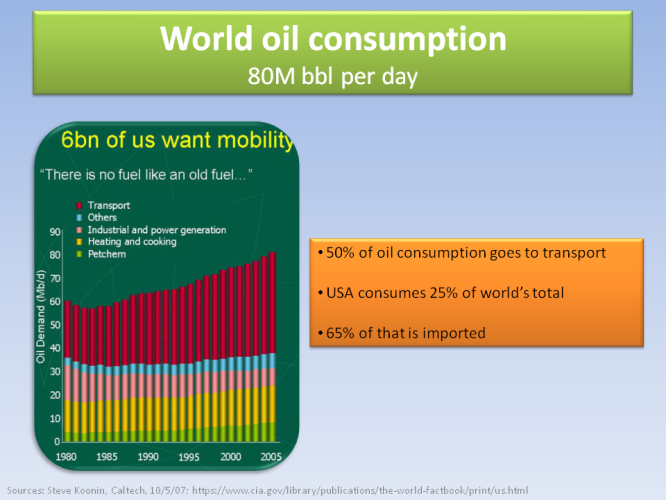
From all the oil consumed in the world, 50% goes directly to transport. 1/4 of all oil in the world is consumed in the US, from which 65% is imported. And the demand is going to rise.
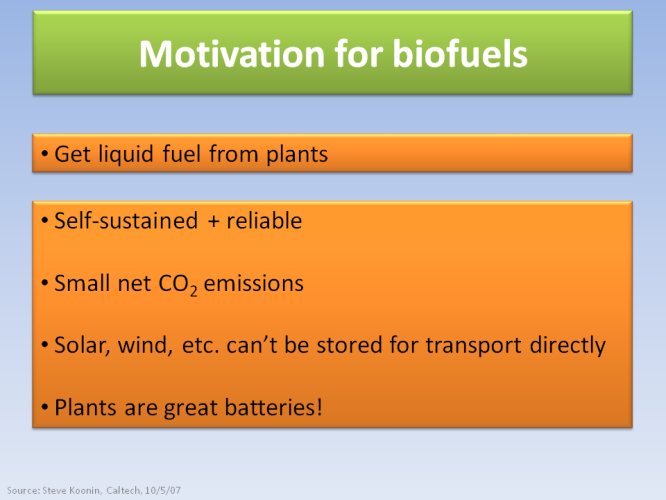
So the idea behind biofuels is to extract the energy stored in plants, and replace a significant amount of the oil demand, mainly for transportation. Biofuels are self-sustained, reliable and they have tiny net CO2 emissions (in principle). They also solve one of the main issues with solar and wind energy, by not requiring extra infrastructure to be transported around the country.
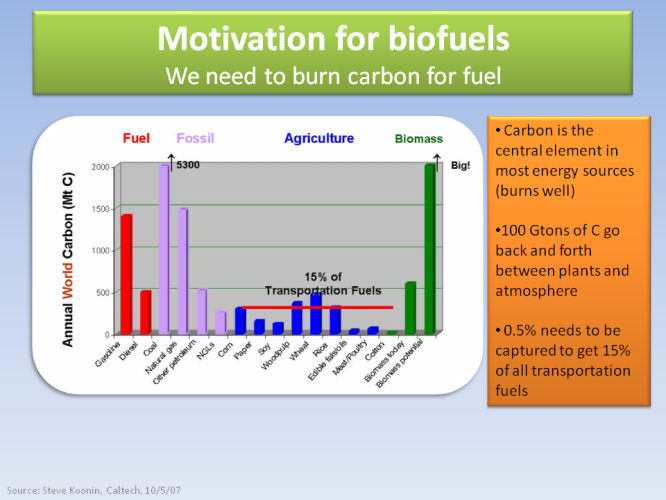
The main reason for biofuels is their potential to cover significant percentage of all transportation fuels. The graph shows the carbon content of various sources of energy, since carbon is what ultimately burns to produce the energy. Gasoline from oil and natural gas are the main sources, as the carbon there originates from plants that have been under the surface of the earth for millions of years. What we need to do is capture 0.5% of the carbon that currently goes back and forth between the active plants and the atmosphere - this will be the equivalent carbon required to cover 15% of all transportation fuels.
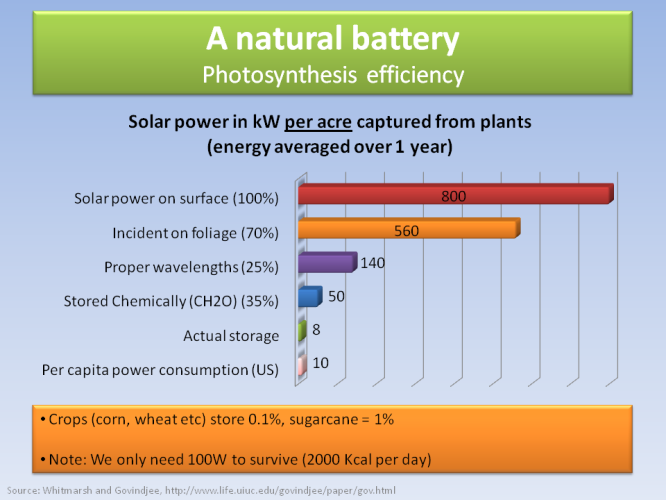
The reason why plants exchange so much carbon is due to their ability to store solar energy, effectively becoming a natural battery. In every acre of land (~4000 m2), averaged over one year, 800 kW of solar power hits the surface. Assuming that 70% of the surface area is covered in foliage, and that 25% of that power is absorbed in the right part of the spectrum, and that 35% of that power is stored in chemical bonds, then the plants store realistically roughly 8 kW of power. Note that the per capita power consumption in the US is 10 kW (other developed countries are not lagging far behind), so we need one acre of plant-battery for every person. Note that we only need 0.1 kW to survive! (2000 kcal per 24 hours).
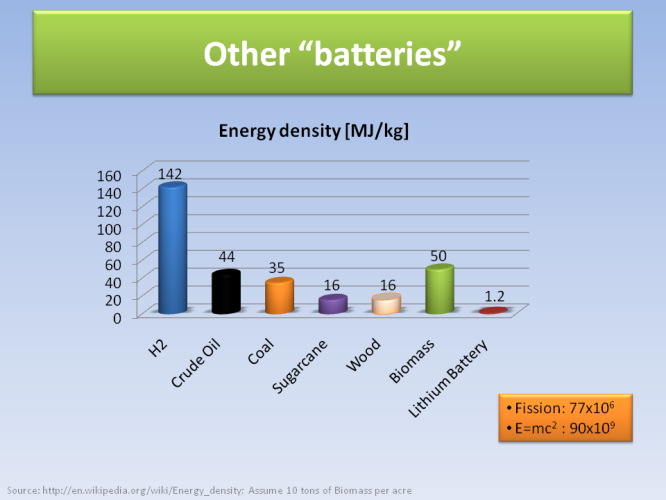
The above slide is probably the most important in all of this work. If we have 1 kg of some material or source of energy, how much energy is stored there?
It turns out that our BEST man-made battery will only store 1.2 MJ (million Joules) for every kg of matter. On the other hand, crude oil and coal store roughly 40 MJ per kg - this is the main reason we keep burning so much oil and coal, since they store tremendous amount of concentrated energy compared to what we can make. It is no accident that it is easy and cheap to simply find that energy and burn it. Burning wood yields roughly 16 MJ, while biomass in general can store 50 MJ per kg (depends on how much foliage we can grow per unit surface area).
For comparison, hydrogen is the best available source by producing 142 MJ per kg - the only problem is that it’s not available freely in nature for us to use it. We have to spend some energy first to make it from water, and then the net energy extracted is not so large. Finally, nuclear fission that is used in nuclear plants produces 77 million MJ per kg of radioactive material (but there are issues of storing the leftovers), while the ultimate limit is the energy corresponding to Einstein’s energy-mass equivalence, where 1 kg of amy material can produce 90 billion MJ of pure energy. (Note that the other forms of energy have nothing to do with Einstein’s equation since they are in addition to that, stored mainly chemically and electrically in the bonds in atoms and molecules).
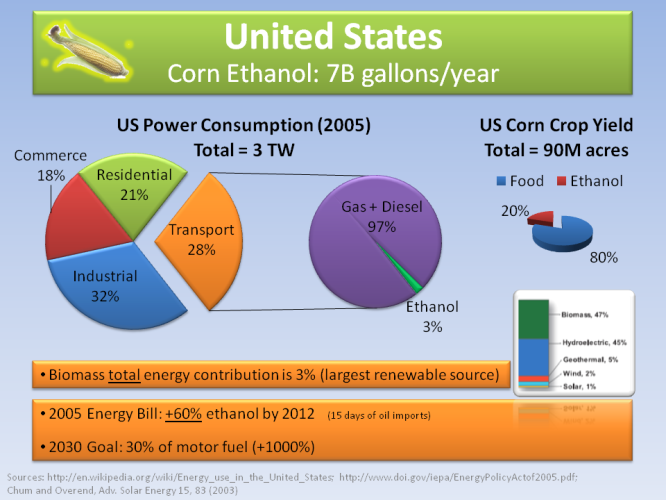
In the US, general biomass right now consists of 3% of all energy consumed, with ethanol being used at 3% of all transpiration fuels, mainly being mixed up to 10% with conventional gasoline sold in gas stations. All ethanol in the US comes from corn, occupying 20% of all corn production.
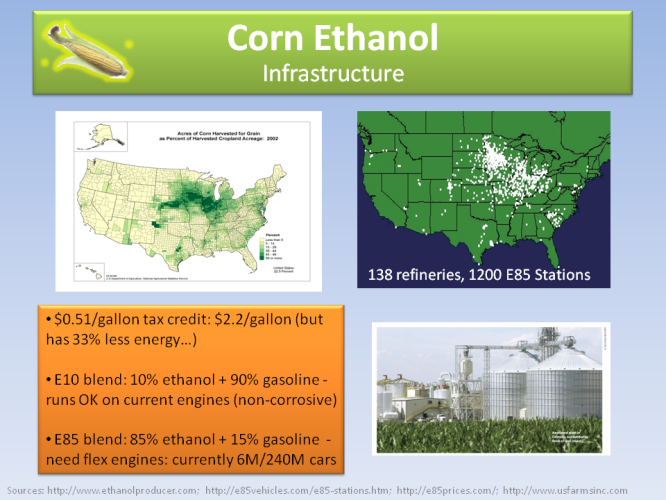
Currently there are 138 corn ethanol refineries and 1200 E85 ethanol gas stations. E85 is a biofuel consisting of 85% ethanol and 15% gasoline, but needs flex engines to run, i.e. specially modified engines designed to burn ethanol. Only 6M cars out of 240M total in the US currently have flex engines (although most people don’t even know it!). E85 is cheaper than gasoline and has 33% less energy output.
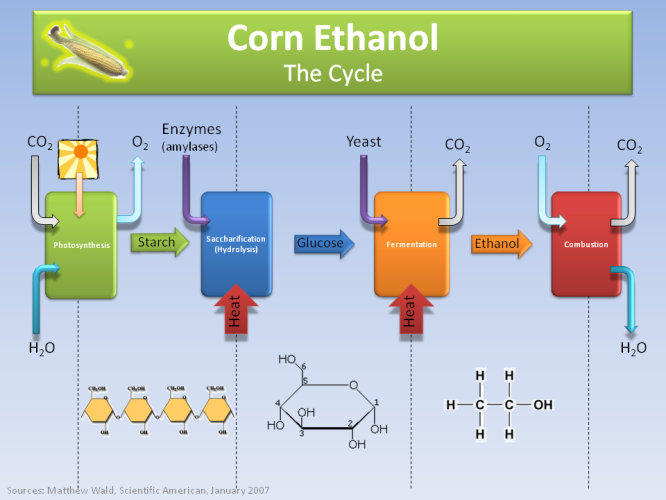
The above graph shows the cycle of producing ethanol from corn. There are two big steps that require a lot of energy in the process, namely hydrolysis (where the starch molecules from plants break down) and then fermentation (where glucose is turned into ethanol by adding yeast). The latter though is exactly the same process we use to produce alcohol!
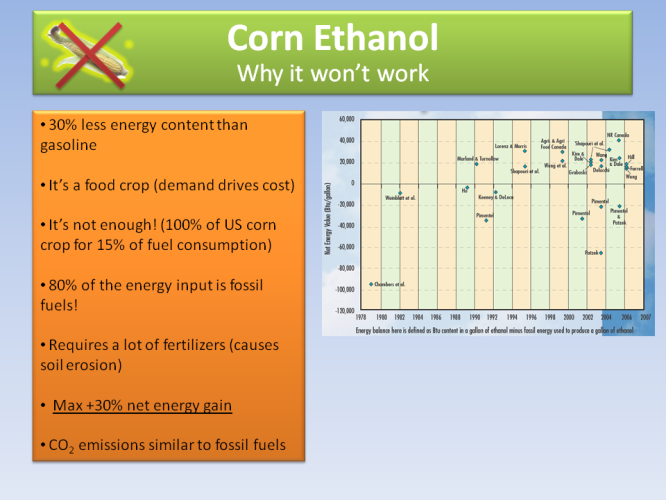
However corn ethanol will never work ultimately, because it requires a lot of fossil fuels energy at various stages in order to produce it. It is not a renewable source, and the maximum net energy gain is at best +30% compared to conventional gasoline. It’s a good start, but not worth it.
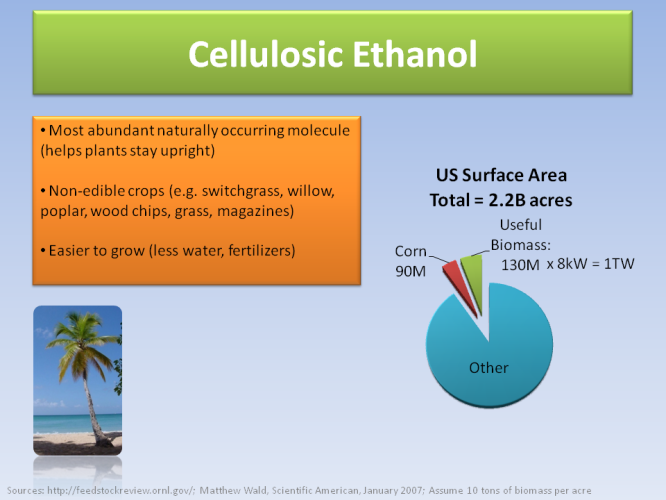
What COULD potentially work is ethanol extracted from cellulose, which is the wall found in all plants (not just corn). This increases dramatically the potential crop sources one can use to get the energy.
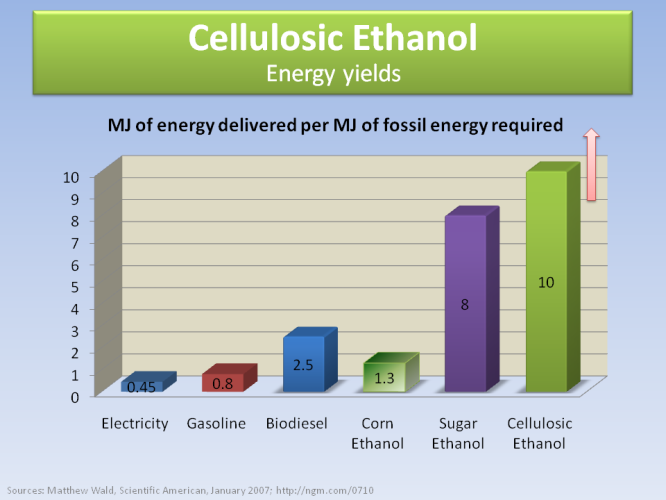
The graph above shows the potential energy that can be extracted from various sources, in terms of the energy required to produce it (so it’s and output/input graph). While electricity and gas have values smaller than one (they have negative balance, i.e. they are not renewable), cellulosic ethanol has the greatest potential of all.
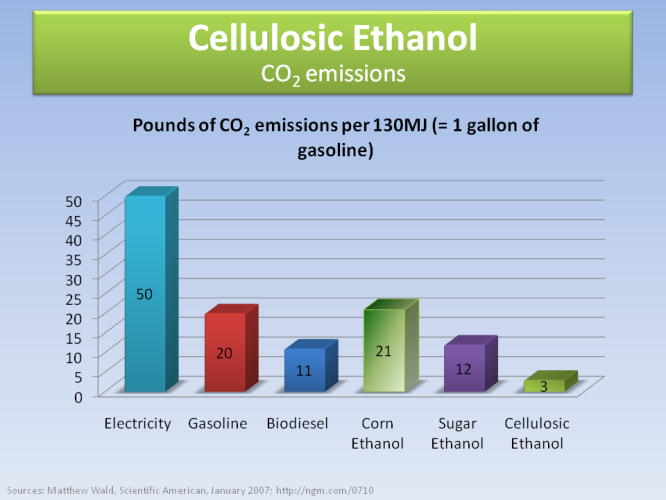
Cellulosic ethanol is also the best option in terms of CO2 emissions, having very few when burned compared to fossil fuels and other biofuels.
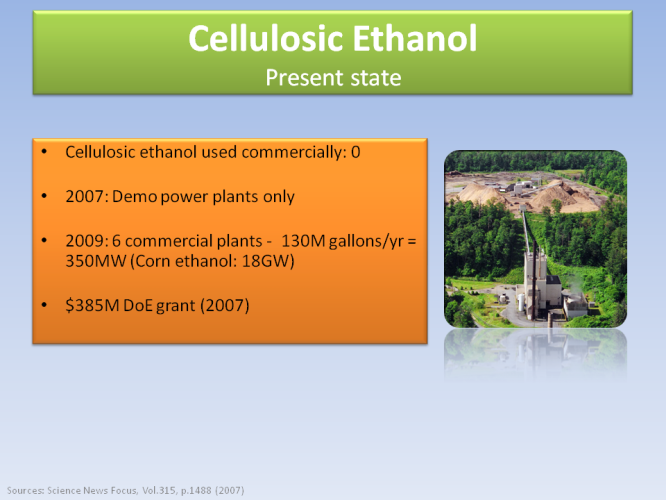
However, this process is still under heavy research. No commercial cellulosic ethanol is produced as of 2007.
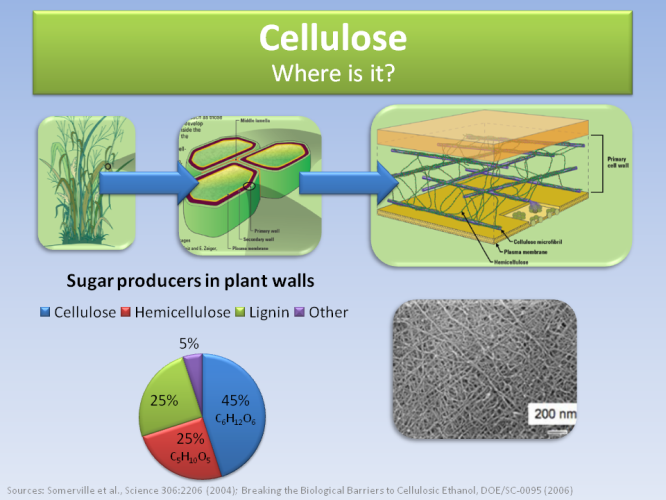
Cellulose comprises 45% of the material in plant walls, as the pictures above and below indicate.
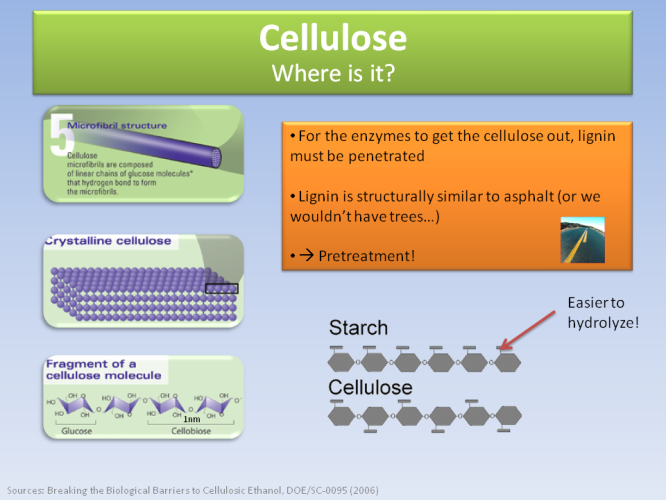
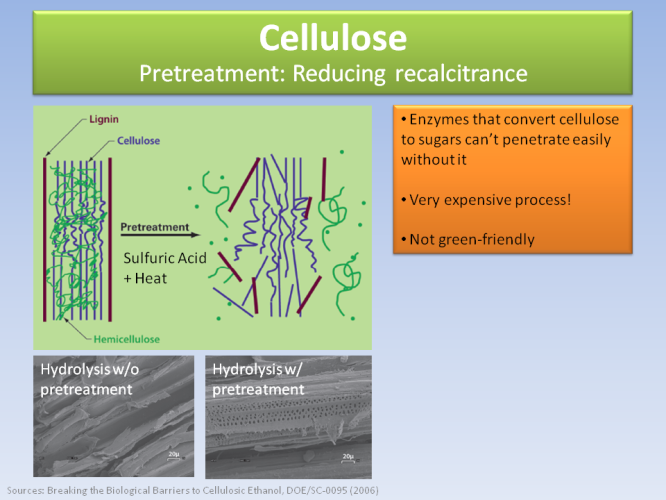
The difficulty is to penetrate the plant wall and get the cellulose out. This is hard and expensive, because it is protected by lignin, which has a chemical composition similar to asphalt (and we don’t know how to break down asphalt…).
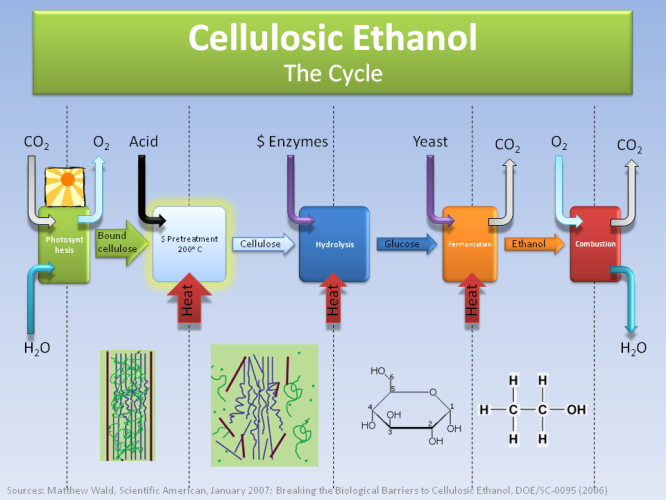
So, until now, it is very expensive and inefficient to extract ethanol from cellulose, despite its great potential.
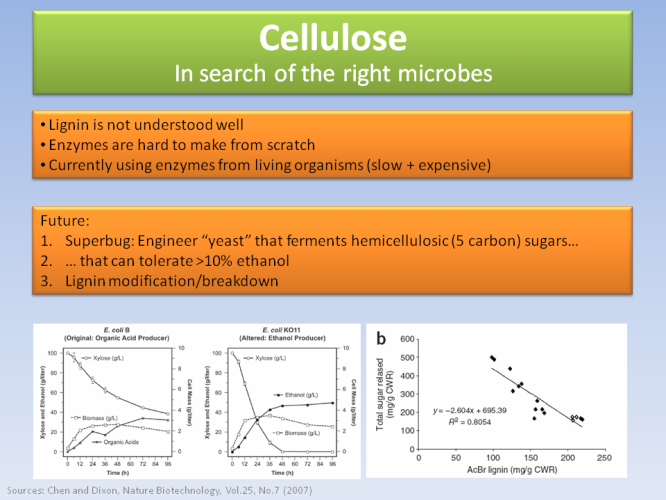
People have been now searching various techniques to break it down more efficiently. When that happens, full-scale production of cellulosic ethanol will quickly spread.

Here are some other biofuel options: Brazil has 40% of their cars running on sugarcane ethanol, and 85% of their cars are flex. This is because Brazil’s climate allows the easier production of the more efficient (compared to corn) growth of sugarcane.
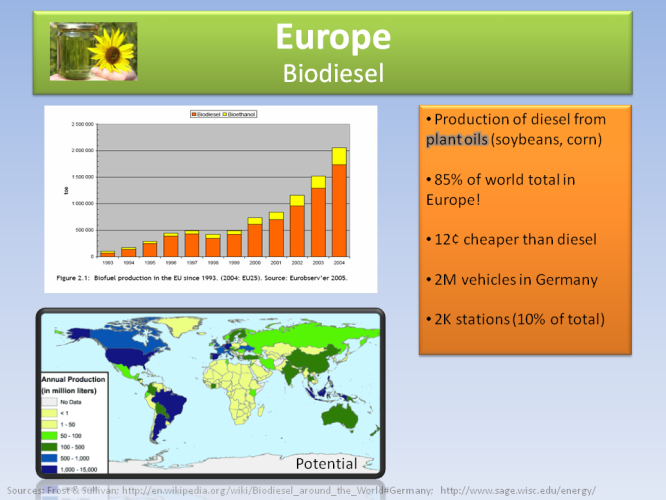
In Europe, BIodiesel (extracted from plant oils) is cheaper than diesel, with 10% of the gas stations in Germany selling it to over 2 million compatible vehicles.
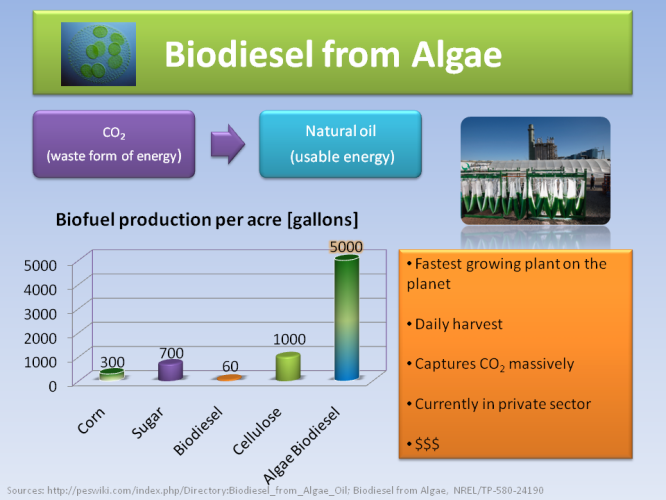
Finally, biodiesel from Algae the greatest potential for a biofuel, because it is the fastest growing plant on the planet, and captures huge quantities of CO2 for storage. However, it is still very expensive to harvest it and remains in the private sector.

The slide above summarizes the main points: corn ethanol is short-term, and cellulosic ethanol is the future, provided that the necessary science techniques are developed.

